Avata Biosciences is a biopharmaceutical firm of the clinical stage with head office located in 6 Stratton street, Mayfair, London W1J 8LD, United Kingdom. The company was established in 2022 and it has developed state-of-the-art cannabinoid-based medicine to treat neurological and psychiatric conditions. It has not taken long before Avata biosciences has become known to be integrating biotechnology, pharmaceutical accuracy, and neuroscience to develop novel, dependable therapies.
The management team of the company is full of scientific and business knowledge. Julian Bryson, the Executive Chairman and co-founder, has a long history of success in spearheading life sciences initiatives. Rupert Haynes, the Chief Executive Officer and co-founder, oversees the worldwide operations and strategy and has years of experience in the area of cannabinoid research and product commercialization. Dr Andrew Saich directs all clinical and regulatory effort as Chief Medical Officer. He is also skilled in neurology and drug development making sure that the therapies developed by the company are of the highest quality in the world. The two have created an organization that is purpose-driven and scientific.
Avata Biosciences is privately owned and is yet to commence trading. Thus, it is not publicly traded with any stock ticker or trading volume. The internal share value however averages at 7.23 per share in the private market. The company has gained more than 45 million USD of early funding by way of seed rounding, starting with an early phase seed round of 5.51 million USD followed by Series A of 40 million USD. These funds fund its research, trials and manufacturing development. Investor confidence is also reflected in the estimated valuation of the company ranging between 150-180million in 2025.
Avata Biosciences has a narrow team of approximately ten employees, who are experts in their field. Its model of operation focuses on productivity of research, lean organization and good partnership with international partners. Such effectiveness helps the company to advance more quickly across the development phases and be financially disciplined.
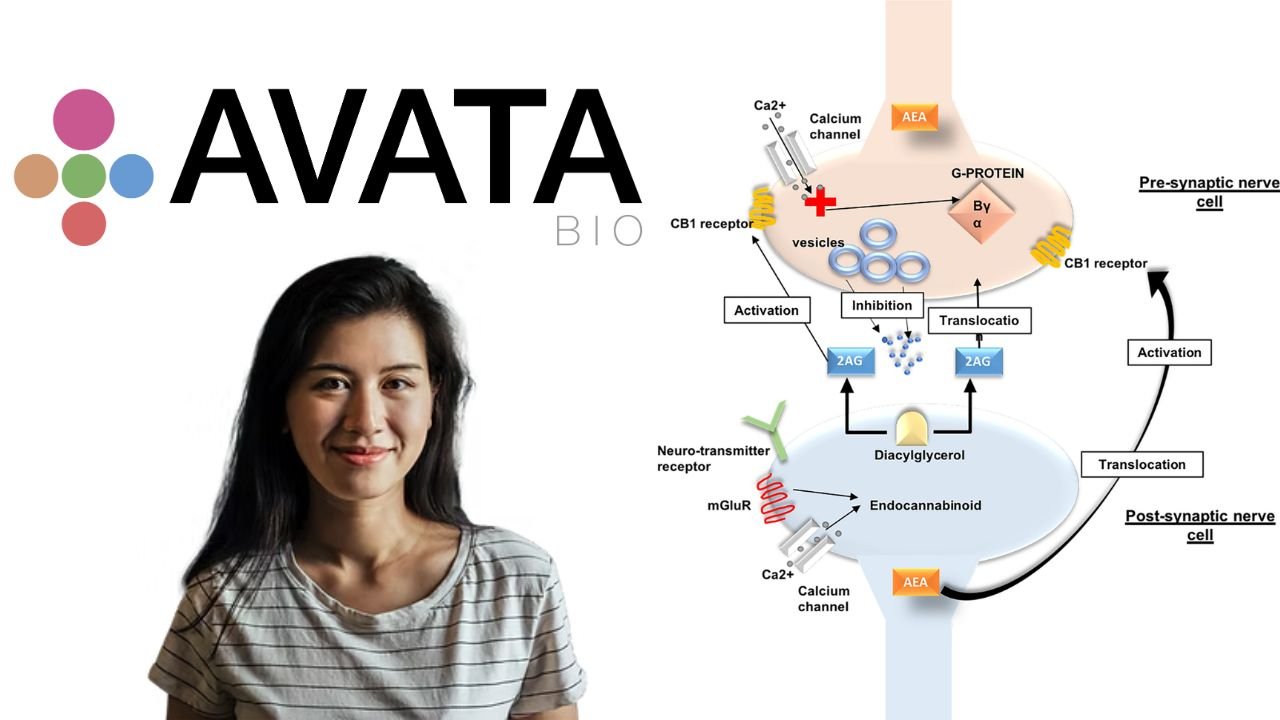
The purpose of Avata Biosciences is evident: to change the process of cannabinoid-based medicines development and prescriptions. Conventional cannabis-based products are also characterized by poor consistency, lack of dose accuracy and inconsistent absorption. This is avoided by synthetic cannabinoid APIs (active pharmaceutical ingredients) used by Avata. These compounds developed in the lab ensure reproducibility, regulatory compliance and purity.
Synthetic production also eliminates defects and inconsistency due to extraction in plants. The same batch is repeatable and therefore the doctors and the patients can depend on consistent results. Avata Biosciences is a pharmaceutical company based in the United States of America, specializing in the production of science-based medicines of pharmaceutical quality through the combination of synthetic chemistry and the development of advanced formulation.
The firm has a central innovation which is the proprietary water-soluble cannabinoids technology. Cannabinoids are usually hydrophobic, which implies that they are insoluble in water. The scientists of Avata have managed to address this issue by developing a procedure that converts these molecules into hydrophilic powders. The innovation enables the use of cannabinoids in solid doses, like tablets and capsules, instead of the conventional oil or liquid forms.
There are several benefits to this change:
- Better bioavailability and increased absorption rate.
- Convenient dosage and uniform strength.
- Increased shelf life and improved transport stability.
- More acceptability by physicians and regulators.
To patients, it will be something familiar and easy to administer that just fits in daily treatment regimens. In the case of the healthcare sector, it entails the production of scalable and standardized cannabinoid drugs which can be produced and distributed worldwide.
AVAT-021 is an adult focal epilepsy drug which is the leading candidate of Avata. Phase 1 trials have already provided favorable safety and absorption outcomes of the therapy. AVAT-022 is another program aiming at treating pediatric epilepsy, and the platform is the same water soluble CBD platform, but modified to children. These two assets are both in clinical development under FDA 505(b)(2) path which accelerates approval of better forms of existing drugs.
The second step the company can undertake is to start Phase 3 trials of AVAT-021 by the end of 2025 and then submit regulatory of AVAT-021 in the US and Europe. Assuming that the outcome is constant, Avata plans to launch its first product in the market by 2028.
A major milestone by Avata Biosciences was achieved in 2025 when it signed a co-development and licensing deal with a leading biotech company in Asia. This joint venture is in Japan and some parts of Asia (not China and India). The acquisition cost is estimated to be more than 90 million dollars USD, with development capital, milestone payments and royalty. It allows Avata to broaden its scope and acquire regional regulatory competencies and keep the global control in London.
This partnership shows that the world has faith in the technology of Avata. It also helps in achieving the long-term vision of the company to provide safe and effective cannabinoid treatment to millions of patients around the world.
- The competitive advantage of Avata is based on five pillars.
- First, the purity of its synthetic cannabinoid ensures accuracy and compliance.
- Second, its drug delivery system is solid, making the treatment of patients and doctors simpler.
- Third, the firm concentrates on the neurological disease that has significant unmet needs such as epilepsy, schizophrenia, and anxiety disorders.
- Fourth, its management team has outstanding credentials in innovation in pharmaceuticals.
- Lastly, it has a stable network of investors to provide solutions and funding.
This combination of strengths will enable Avata Biosciences to become a leader in the next-generation cannabinoid treatment.
Besides the main programs, Avata is investigating into other applications in neurology. The scientists of the company are studying synthetic cannabinoids that can be used in the treatment of schizophrenia, mood disorders as well as chronic pain. They seek to utilize targeted cannabinoid receptors in the regulation of neurotransmitters without psychoactive adverse effects. This study makes Avata a leader in the field of new neurology.
Another new GMP-certified manufacturing plant is also being planned in the UK by the firm. This plant will enable the production of capsules and powders in house, where the quality and compliance with the rules will be controlled in order to commercially supply it. It should start its operations by the year 2026 and is planned to serve both the European and the North American market.
Although privately owned, Avata Biosciences already drew attention of the global influx of scientific rigor and business potential. According to private market information, its stock is priced between 7 and 8 per share, based on the round of funding. The company enjoys financial support of Harwood Private Capital and 5 Horizons Capital, which provided it with adequate financial resources to develop its pipeline to late-stage trials and subsequent commercialization.
Given the current trend of Avata Biosciences, the prospect of future IPO might exist, as soon as the latter passes Phase 3 testing and obtains the appropriate regulatory approval. That would be the step towards becoming a public leader in neurological medicine and a private start-up.
Avata has a scientific methodology that establishes a new standard of the cannabinoid industry. The company fills the gap between natural compounds and pharmaceutical innovation through synthesis of chemical compounds with the help of modern formulation and precision in the clinic. It demonstrates that cannabinoids in designed and tested forms can be safe, effective and universally acceptable.
Until the patients with epilepsy, schizophrenia, or the pain conditions, it is an assurance of a better control, reduced side effects and enhanced quality of life. In the case of the industry, it is a plausible framework of transforming sophisticated natural science into certified remedies.
The prospects of Avata Biosciences are good. The firm will expand its clinical network, find new partners, and launch additional cannabinoid-based neurology and psychiatric drugs. Its technology, leadership and pipeline provide it with a strong base to grow in the future.
Avata Biosciences is a relatively new company formed just a few years ago; however, it is beginning to show that science and strategy can be aligned to provide actual medical advancement. The company is determined to achieve its mission of transforming the science of cannabinoids into effective and accessible treatment to patients globally as it grows closer to success in business.